The biopharmaceutical water for injection preparation system adopts a modular design concept, based on functional units such as pretreatment, RO (Reverse Osmosis) reverse osmosis, EDI (Electrodeionization) continuous deionization, multi-effect distillation, and storage and transfer. It is tailored to meet the water needs of various industries within the pharmaceutical sector, including chemical APIs (Active Pharmaceutical Ingredients), chemical preparations, traditional Chinese medicine decoction pieces, proprietary Chinese medicines, biologics, and liquid preparation. Depending on the specific water quality standards required, the final water production unit is optimized and assembled through various functional modules, ensuring high performance and quality of the entire system. This guarantees that the produced water fully meets or exceeds the water quality standards for water for injection.
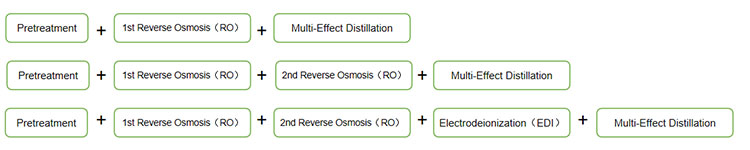
Process flow of water for injection system
Disinfection Methods (Optional):Activated Carbon Pasteurization, CIP (Clean-In-Place) Cleaning System, Ozone Sterilization of Distribution System, Pasteurization of Distribution System, Pure Steam Sterilization of Distribution System.
Provided validation documents:We provide the required DQ (Design Qualification), FAT (Factory Acceptance Test), SAT (Site Acceptance Test), IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification) validation documents for the equipment.
Compliance Standards:In compliance with the standards set by the Chinese Pharmacopoeia, European Pharmacopoeia, United States Pharmacopeia, the pharmacopoeial standards for water for injection, as well as the requirements of the FDA and other relevant standards.












